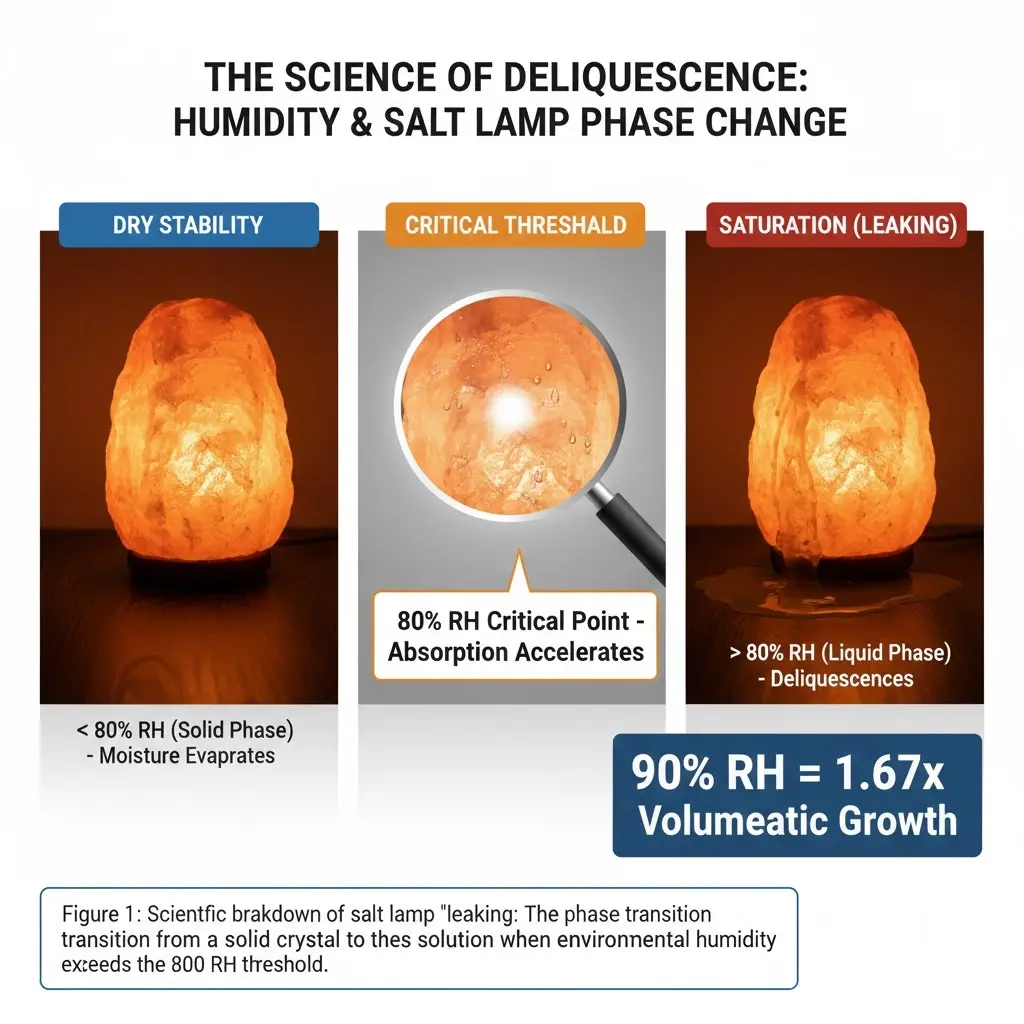
A leaking salt lamp is not melting; it is undergoing deliquescence triggered when relative humidity exceeds **80% RH**. At this critical threshold, inorganic salt crystals shift from solid to aqueous solution, and by **90% RH**, the material exhibits a volumetric growth factor of **1.67**, creating conductive brine that threatens both electrical components and furniture surfaces.
This analysis breaks down the thermodynamics of hygroscopy and outlines immediate remediation protocols for saturated fixtures. We examine **NEMA safety standards** for assessing water-damaged LED drivers, apply **ASHRAE** humidity guidelines to stabilize the environment, and detail **ASTM D-632** specifications for preventing future degradation and maintaining structural integrity.
The Science of Hygroscopy
Salt lamps liquefy when humidity exceeds 80% RH, triggering a phase change from solid to solution. High surface area amplifies this moisture uptake.
Mechanisms of Moisture Uptake
Hygroscopy defines the ability of a material to attract and hold water molecules through absorption or adsorption. This process relies heavily on surface area mechanics. Research indicates that materials with a high Specific Surface Area (SSA)—specifically in the 200-900 m²/g range—drastically amplify moisture uptake capability.
Thermodynamically, water content correlates directly with this surface area via disjoining pressure equations. Instruments like the Hygroscopic Tandem Differential Mobility Analyser (HTDMA) quantify this by passing dried aerosols through controlled humidity steps. These measurements confirm that surface structure dictates exactly how much water a salt crystal holds before saturation occurs.
Deliquescence Thresholds and Phase Data
The visible “leaking” of a salt lamp represents a specific phase transition called deliquescence. This occurs when relative humidity (RH) crosses a critical threshold, forcing the inorganic salt to shift from a solid crystal into an aqueous solution. Research on relevant inorganic salts highlights the exact tipping points where structure collapses into liquid.
- Deliquescence Point: The phase transition from solid to liquid typically triggers at 80% RH.
- Growth Factor: At 90% RH, salt structures exhibit a growth factor of 1.67, indicating rapid volumetric expansion.
- Particle Expansion: Dry particles measuring 20-500 nm can swell to over 1000 nm when fully wet.
- Precision: Labs verify these shifts with humidity control precision of ±1% using HTDMA scans.
Why “Leaking” is Normal
“Leaking” confirms authenticity. Through hygroscopy, salt crystals absorb airborne moisture, which condenses into liquid brine when the lamp cools or humidity exceeds the material’s deliquescence point.
| Hygroscopic Phase | Relative Humidity (RH) | Physical Result |
|---|---|---|
| Dry Stability | < 80% | Moisture evaporates; crystal remains solid. |
| Deliquescence Point | 80% | Transition threshold; absorbtion accelerates. |
| Saturation (Leaking) | 90% | Phase change to aqueous solution (1.67x growth). |
The Hygroscopic Mechanism
Hygroscopy defines the chemical capability of salt crystals to attract and hold water molecules from the surrounding atmosphere. This process relies on the material’s specific surface area (SSA). Research indicates hygroscopic powders possess an SSA ranging from 200 to 900 m²/g, creating massive opportunities for adsorption. Water molecules, which have a cross-section area of approximately 0.125 nm² at room temperature, latch onto these porous surfaces.
Genuine Himalayan salt exhibits this porosity naturally. Synthetic imitations lack the complex crystalline structure required to achieve high SSA values, meaning they rarely attract enough moisture to leak. If your lamp stays perfectly dry in a humid room, it is likely a fake.
Saturation and Thermal Equilibrium
“Leaking” occurs when the rate of absorption outpaces evaporation. When the lamp is lit, the heat creates a dry cycle, evaporating moisture back into the air. However, when you switch the lamp off, the salt continues to attract water without the heat source to drive it away.
- Deliquescence Point: Inorganic salts hit a critical transition at roughly 80% RH. Below this threshold, they remain solid.
- Phase Change: At 90% RH, salt exhibits a growth factor of 1.67. The crystal structure expands and dissolves into the absorbed water.
- Visible Result: This creates a brine solution that pools at the base, often mistaken for a leak in the physical lamp.
Physics dictates this reaction. Thermodynamics link water content directly to the available surface area and humidity levels. The pooling water is simply the salt attempting to reach equilibrium with a humid environment.
Ideal Humidity Levels
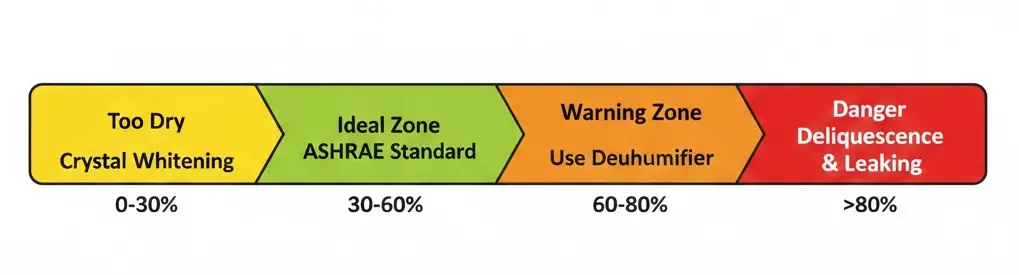
Maintaing 30–60% RH prevents hygroscopic leaking while aligning with human comfort. Levels above 50% trigger deliquescence, requiring immediate environmental control to stop moisture accumulation.
The Safe Zone: 30-60% Relative Humidity
Standard engineering guidelines align material stability with human health. ASHRAE recommends a baseline of 30-60% RH for occupied spaces. Within this window, salt products remain dry, and the internal heat from the bulb effectively manages minor moisture absorption without overwhelming the material.
For maximum longevity, aim for the narrower target of 40-60% RH. This range balances material value retention with occupant comfort. Data from Class 3K5 standards (BS EN 60721-3-3) supports this, indicating that deviations outside standard ranges often require auxiliary heating to prevent saturation or drying. If you drop below 30%, the environment becomes uncomfortably dry; if you exceed 60%, you are fighting physics.
Critical Thresholds: Deliquescence Risks Above 50%
Hygroscopic materials do not just get wet; they pull water from the air until they dissolve. This process, known as deliquescence, becomes visible as “crying” when humidity crosses specific thresholds. Once moisture accumulation exceeds evaporation rates, the structural integrity of the salt compromises.
- <45-50% RH: The critical upper limit to prevent visible leaking. Below this point, corrosion risks remain low.
- >60% RH: Vapor pressure equilibrium breaks down. Moisture accumulates faster than the lamp can evaporate it, significantly accelerating corrosion risks.
- >65% RH: Environmental controls become mandatory. You must employ dehumidifiers or HVAC adjustments, as ambient conditions consistently exceed the material’s capacity to self-regulate.
Source Premium Himalayan Salt Bricks Direct from the Factory

Immediate Fixes for Wet Lamps
Disconnect power immediately. Per NEMA standards, replace water-damaged drivers and sockets; never recondition them. Only the salt block and intact wiring are salvageable.
| Component | Condition Risk | Standard Action (NEMA/NEC) |
|---|---|---|
| LED Drivers / Ballasts | Water Exposure | Replace (Do not recondition) |
| Wiring / Cabling | Ends Dry, Insulation Intact | Recondition |
| Fixture Mounting | Wet Location | Mount 4ft+ above ground |
| Industrial Fixture | Splash/Washdown | Must be rated IP65 or higher |
Emergency Disconnection and Hazard Assessment
Brine is highly conductive, turning a “crying” lamp into a significant shock hazard. Your first move is to cut the power to prevent short circuits. Once safe, you must assess the damage based on industry safety standards rather than attempting to dry out complex electronics. NEMA protocols are strict here: electrical components compromised by water often cannot be saved.
- Immediate Power Cut: Disconnect the luminaire instantly. Do not handle the wet fixture while it is energized.
- NEMA Classification: Discard any ballasts or LED drivers exposed to water. These components are non-repairable and pose future fire risks if re-used.
- Wiring Assessment: Inspect the cord assembly. You may recondition the wire only if the ends and insulation are undamaged and show no signs of brine intrusion.
Drying Protocols and Component Replacement
The leakage occurs because the salt reaches its deliquescence point at approximately 80% Relative Humidity (RH), causing a phase change from solid to aqueous solution. While you can dry the stone, the hardware requires rigorous inspection or upgrading to prevent recurrence. Standard fixtures often fail under the corrosive stress of salt brine.
- Moisture Removal: Separate the bulb assembly and wipe the salt block completely dry. The housing must be moisture-free before you consider reassembly.
- Upgrade Specifications: If high humidity is constant (>80% RH), switch to fixtures rated IP65 (jets) or IP67 (immersion) to block ingress.
- Corrosion Check: Inspect socket contacts for salt corrosion. Replace the unit if degradation is visible, as salt spray accelerates failure in standard copper fittings.
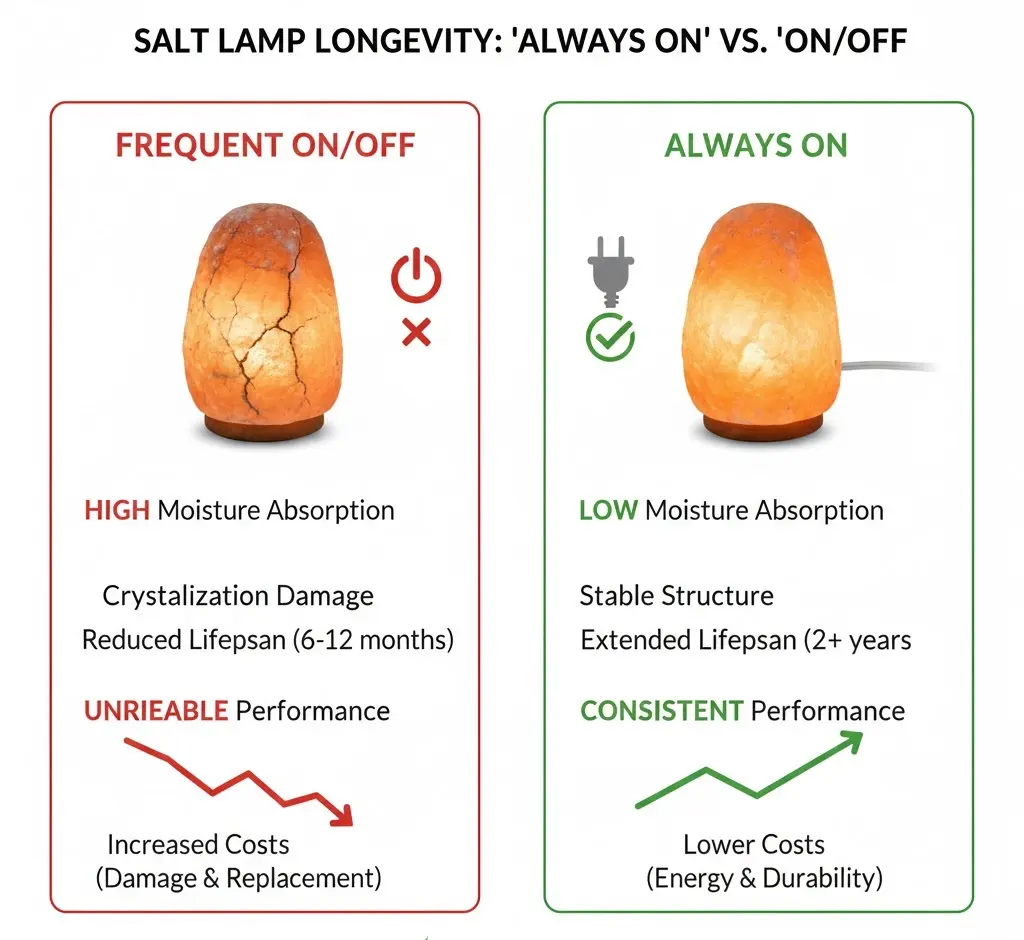
Long-term Maintenance: “Always On”
Avoiding full shutdowns prevents salt degradation. For storage, seal containers to keep surface moisture under 0.2% and maintain bulk density per ASTM D-632 standards.
Continuous Operation and Idling Protocols
Full shutdowns often cause more damage than continuous wear. Industrial salt baths face significant viscosity and surface tension issues when cycling through ranges of 400-1000°F. To maintain bath integrity, operators should avoid cold shutdowns in favor of idling protocols. Reducing the temperature allows a hard insulating crust to form on the surface, which conserves energy while keeping the underlying chemistry active and ready for ramp-up.
- Burner Tube Coverage: Maintain constant salt coverage over tubes during melt-down to prevent overheating.
- Manual Agitation: Perform manual pushing or hoeing during operation to ensure uniform heat distribution.
- Make-up Additions: Introduce fresh salt in controlled batches (e.g., 500 lb drums) to offset drag-out losses.
Storage Specifications and ASTM Standards
Salt is naturally hygroscopic. If you expose it to ambient humidity without sealing, it absorbs water, leading to “crying” (moisture exudation) and rock-hard clumping. Effective long-term storage requires strict adherence to ASTM D-632 standards to prevent sifting and moisture ingress. Keep drums tightly closed and minimize handling delays to sustain flowability.
- Surface Moisture: 0.05% target (0.2% maximum) to prevent caking.
- Bulk Density: 1025-1185 g/l (64-74 lb/cu ft) for stable stockpiling.
- Water Insolubles: 0.01-0.15% via saturated brine washing.
- Particle Size Distribution: 10-27% retained on USS 20-70 Mesh.
Preventing Furniture Damage
Salt lamps absorb moisture, creating corrosive brine that warps wood and stains varnish. Always use non-porous barriers or treated coasters to isolate the base from delicate surfaces.
Understanding Hygroscopic Risks to Wood
Furniture finishes rarely withstand prolonged contact with the saline solution generated by salt lamps. The core issue lies in the hygroscopic nature of the salt, which attracts water molecules from the air. Research indicates that inorganic salts reach a deliquescence point—often around 80% relative humidity (RH)—where they undergo a phase change from solid to aqueous solution. At 90% RH, the material expands with a growth factor of 1.67, resulting in visible liquefaction or “crying.”
This liquid is not pure water; it is a concentrated brine. Wood is naturally porous, and when this saline solution pools at the base of a lamp, the furniture absorbs it rapidly. This interaction causes the wood fibers to swell (warping) and chemically reacts with varnish, leaving permanent white rings or discoloration that standard cleaning cannot remove.
Protective Barriers and Placement Solutions
Isolating the hygroscopic base from your furniture provides the only reliable defense against corrosion. Since humidity control operates within precise margins (often ±1%), rely on physical barriers rather than environmental control alone.
- Custom Wooden Coasters: Use treated Basswood coasters to separate the lamp base from delicate surfaces, as this wood offers better humidity resistance.
- Non-Porous Materials: Place lamps on ceramic, glass, or stone bases in environments where humidity frequently exceeds the 80% deliquescence threshold.
- Moisture-Resistant Placemats: Utilize barriers specifically designed to block liquid transfer if the lamp begins to sweat during high-humidity cycles.

Storage Tips
Effective storage demands moisture levels between 1.5% and 4% to stop deliquescence. Covered barns with impervious pads outperform sheeted stockpiles and ensure ASTM D632 compliance.
Environmental Control and Containment Strategies
Physical infrastructure directly impacts salt stability and application efficiency. You must prioritize permanent covered storage, such as salt barns, over temporary sheeted stockpiles. Covered structures provide superior condition control, which keeps the material dry and flowable. This allows operators to reduce spread rates to <10 g/m², saving material costs compared to wet, clumping salt.
- Foundation: Install impervious pads and retention ponds to prevent leaching and isolate stocks from groundwater.
- Covering Protocols: Enforce strict covering to stop moisture ingress, as excess humidity triggers deliquescence (crying) and causes tunneling in hopper machinery.
Technical Metrics: Moisture Limits and Piling Physics
Stable storage relies on precise engineering data rather than guesswork. Maintaining the correct moisture content prevents the phase changes that lead to solidification. For dry salting, keep moisture between 1.5% and 4%. If you use pre-wetted sodium chloride, maintain levels <4% to avoid clumping.
- Purity Standards: Adhere to ASTM D632 (Type 1, Grade 1), requiring ≥95% NaCl to eliminate handling issues caused by impurities.
- Stockpile Geometry: Design piles using an angle of repose of 40-44° to prevent collapse.
- Density Calculations: factor in a bulk density of 1200 kg/m³ for accurate volume and space planning.
Final Thoughts
Leaking isn’t a defect; it is physics punishing you for high humidity. Without heat to drive off moisture, your salt lamp inevitably dissolves into a corrosive brine puddle on your furniture.
Keep the bulb running 24/7 to maintain thermal equilibrium and prevent saturation. If you must shut it down, seal the lamp in an airtight bag immediately to stop moisture ingress.
Experience the quality firsthand with our low-threshold trial order – a risk-free way to test these high-margin products in your market. Get in touch to start your trial order today!
Frequently Asked Questions
Is the salt lamp melting when it starts to leak?
No. Sodium chloride (table salt) requires a temperature of 801°C to melt. You are observing deliquescence, a process where the salt absorbs atmospheric moisture until it saturates and dissolves into liquid brine at room temperature.
How can I effectively dry out damp salt?
You need consistent heat to remove moisture from saturated salt. While turning the lamp on helps with maintenance, technical drying standards for sodium chloride dictate heating the material to 110°C ± 5°C to achieve a constant dry weight.
Can I replace the bulb with an LED?
Yes, but with a trade-off. LED bulbs fit standard sockets and provide high efficacy (up to 150 lm/W). However, because LEDs emit minimal heat, they often fail to generate enough thermal energy to keep the salt crystal dry in humid environments.






